The focus of our lab is understand how cellular cargos are delivered to the right place at the right time. Intracellular transport is a critical feature of cell physiology, and is largely mediated by a small group of molecular machines called motor proteins. We pay particularly close attention to one such molecule called dynein, and how this motor is regulated to perform its myriad functions during cell division.
Research :
How do dynein motors properly move the mitotic spindle during mitosis?
In many tissue types, cell fate and consequent tissue organization are dictated by the
orientation of the mitotic spindle with respect to the cell boundaries. During such processes as organismal
development and tissue homeostasis, spindle orientation dictates the plane of cell division, and thus whether a
cell divides symmetrically or asymmetrically. Symmetric stem cell divisions result in two identical stem cells, whereas a
switch to asymmetric division results in one stem cell and a differentiated cell. Thus, proper coordination of spindle
position with the particular needs of a tissue or cell type is critical during numerous biological processes. Improper
spindle orientation can compromise asymmetric stem cell divisions, impair differentiation, and lead to defects in tissue
development and homeostasis. In fact, unchecked symmetric and asymmetric divisions have both been directly linked to cancer
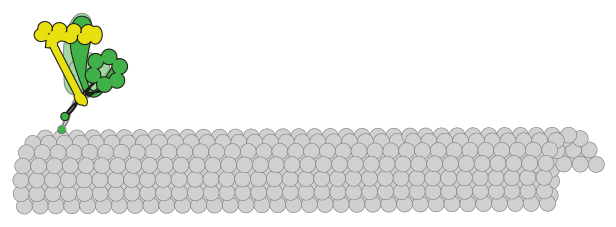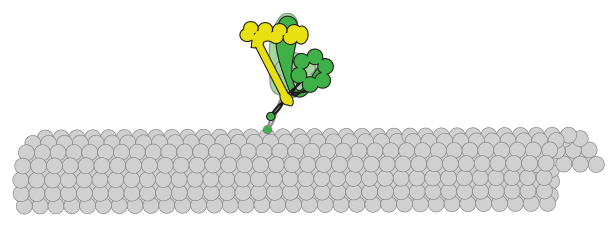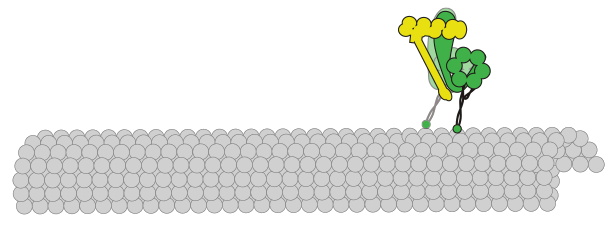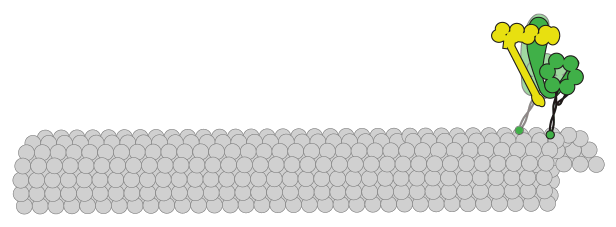
initiation and progression. A key effector of spindle orientation is the molecular motor dynein. This motor is anchored at
the plasma membrane from where it orients the spindle through precisely tuned interactions with microtubules. It is unclear
how cortically anchored dynein motors perform this function with appropriate directional and temporal control to achieve
proper tissue specific functions. The lack of such information presents an impediment towards the development of effective
therapies that may prevent or reverse defects in tissue organization that can lead to developmental disorders or cancer.
To advance our understanding of this process, our lab uses the simple model organism budding yeast – in which dynein and many of its
regulators are highly conserved – and a combination of in vivo, in vitro, and biophysical methods to determine the mechanisms by
which dynein is activated to perform its spindle orientation function, and regulated to achieve appropriate directionally biased
spindle movements.

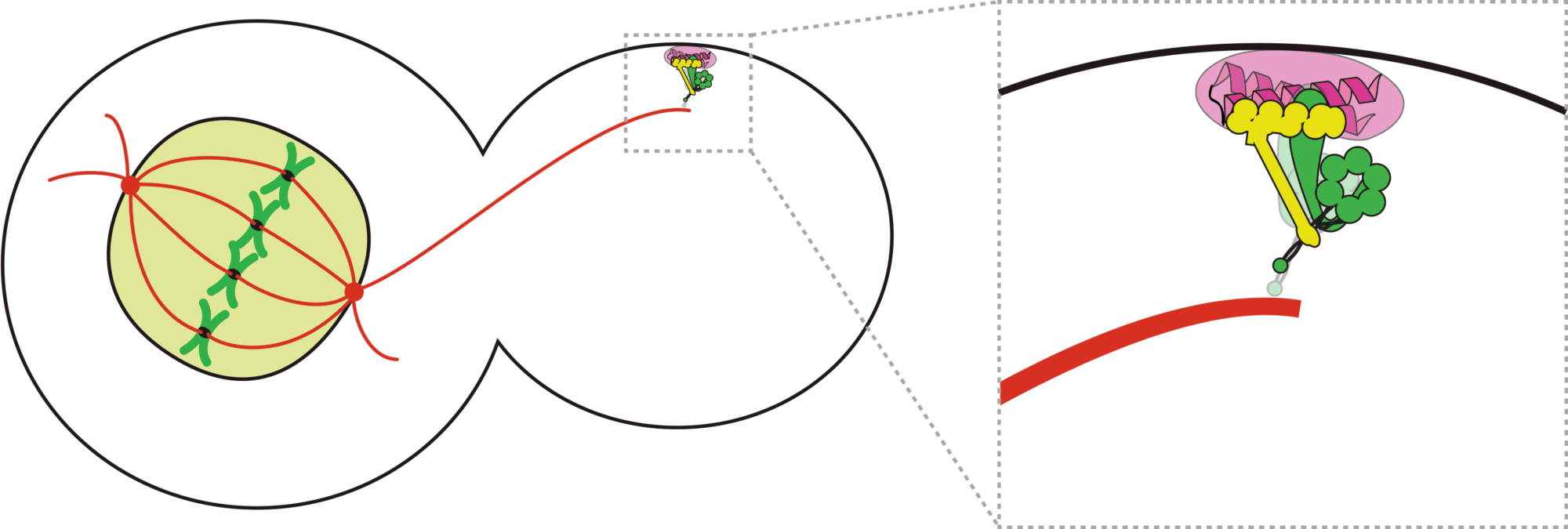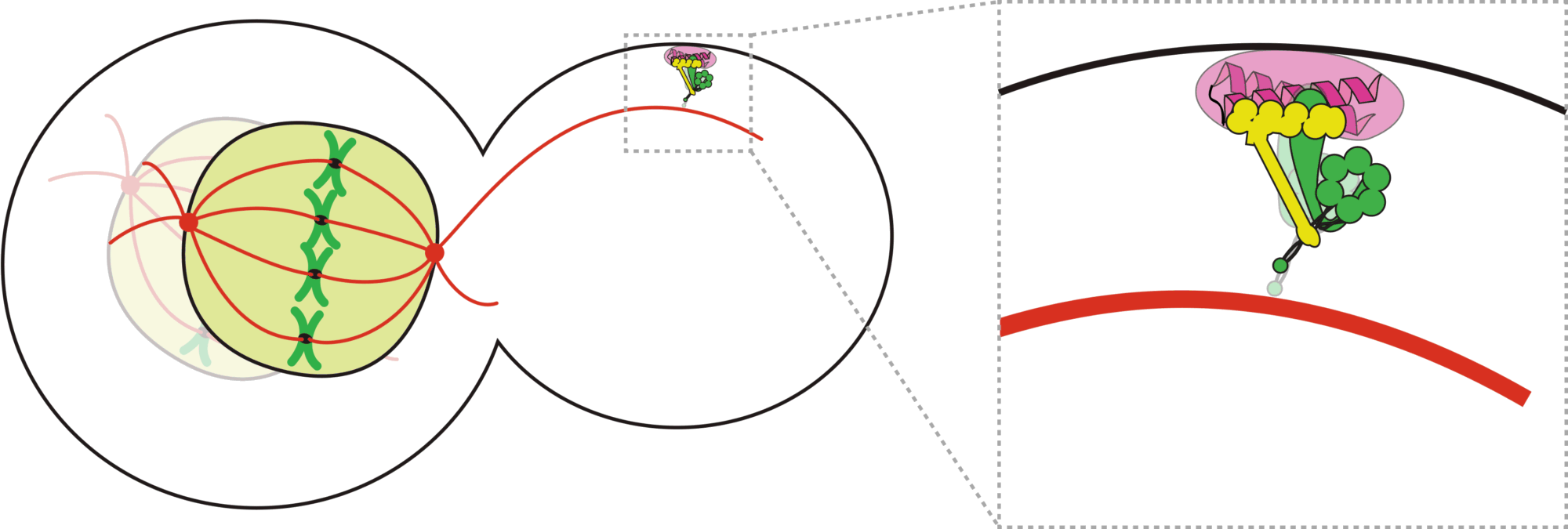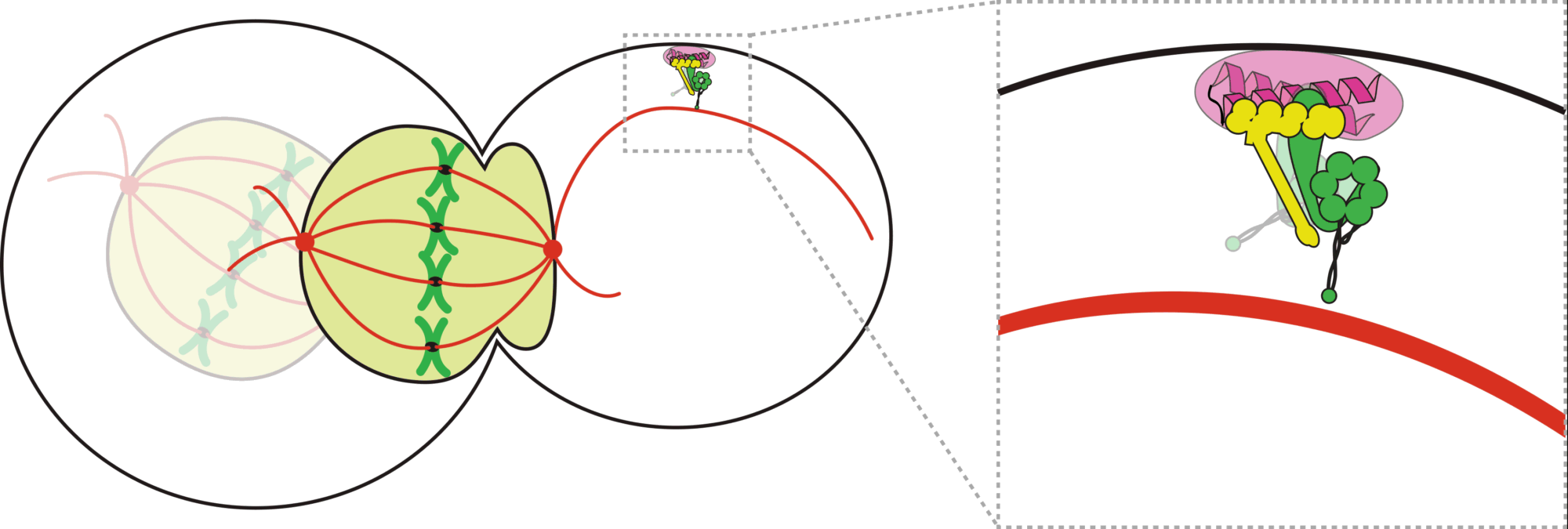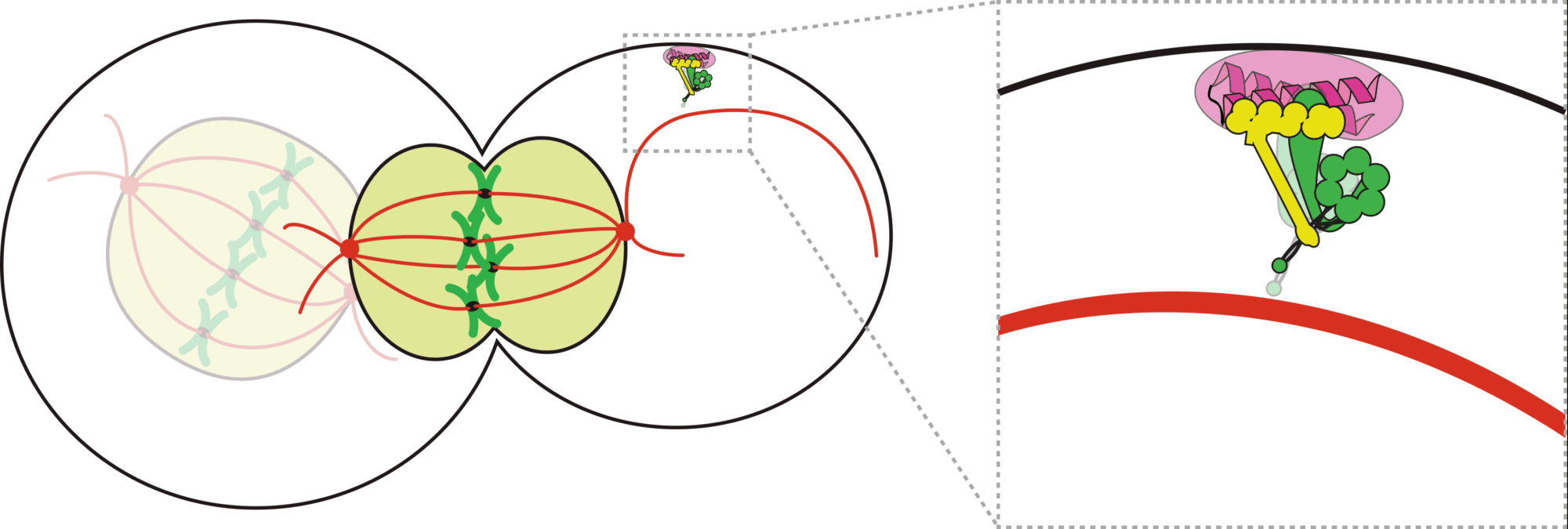
Animation depicting a dynein mediated spindle movement in budding yeast.
What is the molecular basis for motor neuron disease?
In addition to spindle orientation during mitosis, dynein is also responsible for the transport of various cellular cargoes in neurons. For instance, dynein motors transport vesicles and unfolded proteins from the axon terminus toward the cell body. Defects in dynein-mediated cargo transport lead to situations in which cargo accumulate near the axon terminus. In particular, unfolded proteins that accumulate are prone to aggregate and can compromise cell viability, most notably in motor neurons. Thus, defects in dynein-mediated transport have been directly linked to a host of motor neuron diseases, including spinal muscular atrophy, congenital muscular dystrophy, and amyotrophic lateral sclerosis; however, the molecular bases for dynein dysfunction in affected individuals are unknown. One of the goals of our laboratory is to use a combination of cell biological, biochemical, and single molecule approaches to define the molecular defects that are associated with various types of motor neuron diseases.
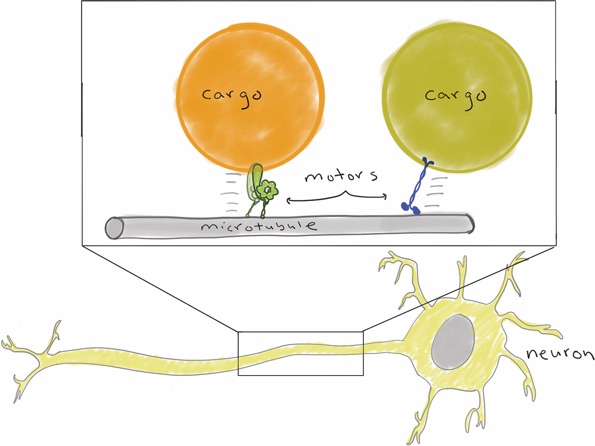
Schematic depicting motor-mediated transport of various cargoes within a neuron.
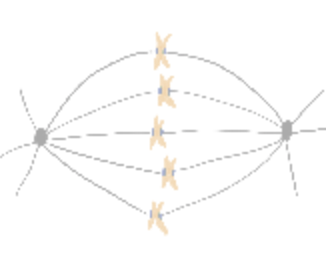
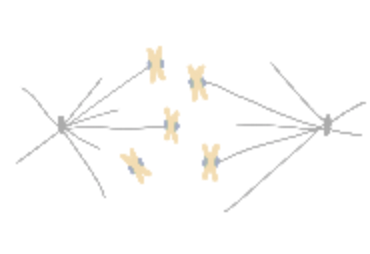
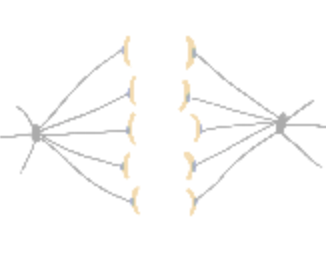
How do cells properly segregate their genetic material?
The goal of this project is to understand how cells accurately and reliably segregate their genetic material
during cell division. Cells have evolved highly complex and elegant mechanisms to ensure that chromosome segregation errors
occur with remarkably low frequency. During cell division, the mitotic spindle functions to separate sister chromatids through
direct attachments between filamentous microtubules and kinetochores, the latter of which are large macromolecular assemblies
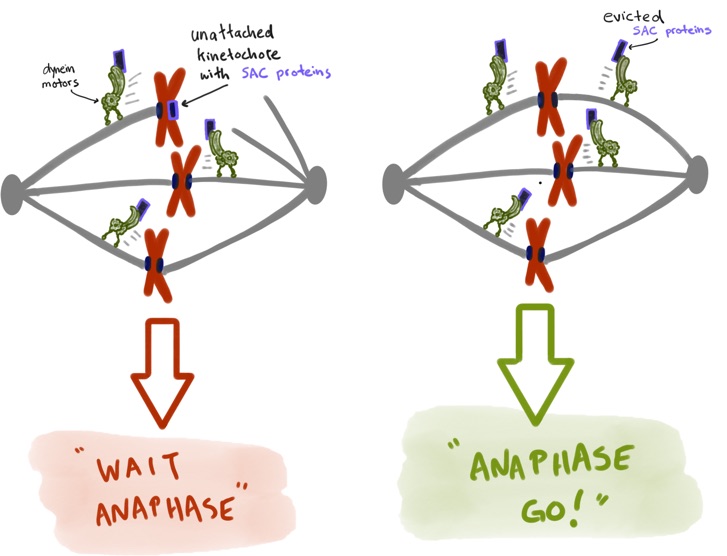
built upon specific genomic regions. To prevent errors during this process, cells employ a fail-safe mechanism called the spindle
assembly checkpoint (SAC), which prevents mitotic exit until all chromosomes have established proper kinetochore-microtubule
attachments. The effectors of the SAC accumulate on improperly or unattached kinetochores, and consequently transmit a
“wait anaphase” signal. Upon establishment of proper attachments, these SAC proteins are evicted from kinetochores, which
leads to silencing of the inhibitory signal, and consequent initiation of anaphase and progression through mitosis. How microtubule
attachment status is translated into SAC protein depletion from kinetochores and consequent SAC silencing is a major unanswered
question in cell biology. A key effector of this process is the microtubule motor protein dynein, which transports SAC proteins
away from kinetochores in response to proper microtubule attachment; however, a clear understanding of the role for dynein in this
process is lacking. Furthermore, how attachment status is communicated to activation of dynein-mediated removal of SAC effectors
is not understood.
By combining an in vitro approach with in-cell high- and super-resolution fluorescence microscopy, we will examine the
following key questions: (1) What is the role of dynein in eviction of SAC effectors from attached kinetochores? And (2)
What is the biological signal that initiates dynein-mediated eviction of SAC effectors from attached kinetochores?
This project is a collaborative effort with our good friends in the DeLuca Lab
Home Research Publications Movies